0 Sterilization Process & Equipment Validation: Circle of Compliance
- The Steam Pulse
- by Kevin Peacock
- 14-06-2024
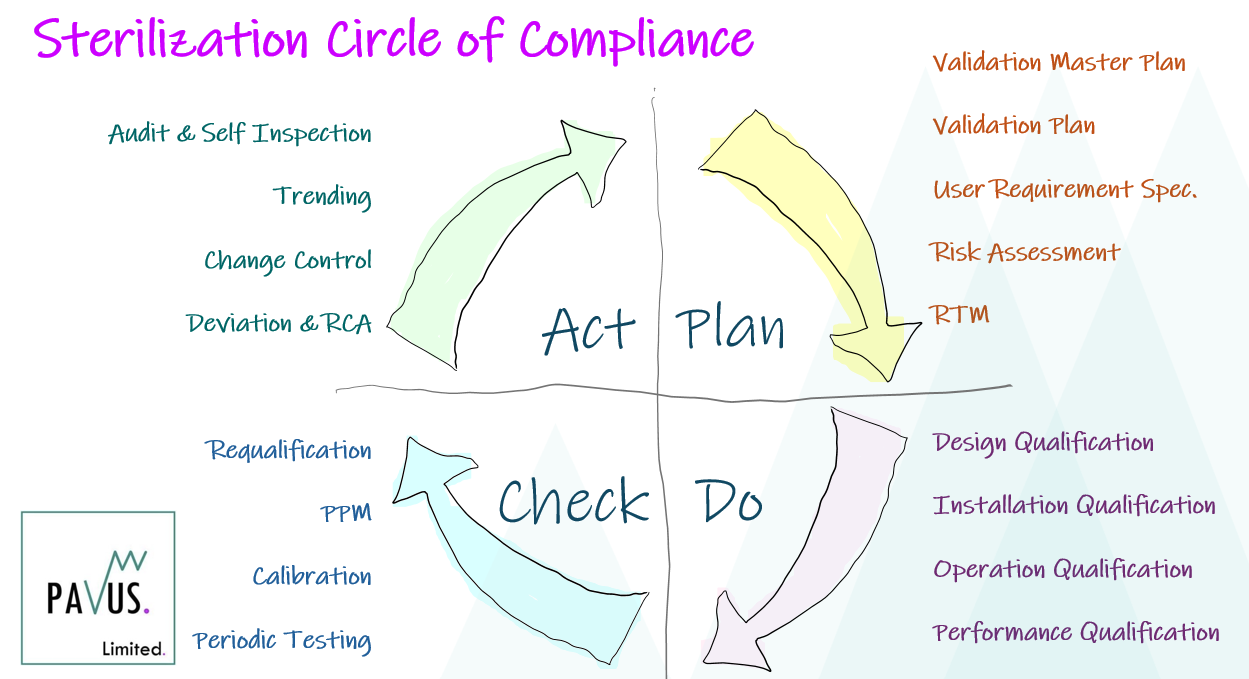
Many are familiar with the Validation V-Model and the Deming Wheel.
Like the Reusable Instrument Cycle in HTM01-01 [1] I like to visualize the Validation Lifecyle as a circular journey, moving through each of the 4 Stage Gates of Plan-Do-Check-Act.
Within each stage there are discreet activities that we must complete and over the coming weeks I will go through each stage and discuss the essential elements that one must consider in the Commissioning, Validation, Routine Monitoring & Control of your sterilization process or equipment.
For me, the validation exercise is not a journey along a one way street, culminating in annual Requalification testing, but a circle of compliance measures, checks and balances put in place to maintain our compliance position and sustain a repeatable and reliable service.
[1] HTM01-01: Part A - Management & Provision, Clause 1.5.
